Introduction
Paweł Rubiś MD, PhD
Rare diseases of the heart (cardiomyopathies)
According to the definition, proposed by the Working Group on Myocardial and Pericardial Diseases of the ESC, cardiomyopathy is “a myocardial disorder in which the heart muscle is structurally and functionally abnormal, in the absence of coronary artery disease, hypertension, valvular heart disease and congenital heart disease sufficient to cause the observed myocardial abnormality” [1]. Therefore, whenever the cardiomyopathy is suspected in an individual patient, an active and comprehensive exclusion of those four common causes of heart damage and failure should be performed at the beginning of diagnostic process.
Clinically-orientated classification traditionally distinguish four distinct structural and functional cardiomyopathy phenotypes, such as dilated (DCM), hypertrophic (HCM), restrictive (RCM), and arrhythmogenic right ventricular cardiomyopathy (ARVC) [1]. Although very useful on the daily basis, it should be acknowledged that frequently cardiomyopathy overlap or mimic one other, e.g. end-stage (dilated) HCM, or ARVC with predominantly left ventricular involvement. Moreover, cardiomyopathies are further sub-classified into familial and non- familial forms. In order to confirm familial cardiomyopathy it is necessary to diagnose either the same or similar (considerable differences in age and gene penetration) cardiac phenotype in at least two family members. Most familial cardiomyopathies are monogenic disorders. Non-familial cardiomyopathies are defined by the presence of the disease in the index patient and the absence of the disease in other family members [1].
Dilated cardiomyopathy
The phenotype of DCM is established by means of imaging studies – echocardiography being the most common. DCM is characterized by left ventricular (LV) dilatation and usually severe impairment of systolic function and associated changes in diastolic function.
The most reliable data on the epidemiology of DCM comes from a relatively old study from an Olmsted County, Minnesota conducted between 1975 to 1984, which estimated DCM prevalence at 35.5:100,00 inhabitants (1:2,700). The annual incidence of DCM is approximately 5 and 8 per 100,000 subjects in the general population [2]. Although DCM may occur at any age, in great majority of cases it affects adolescents and young adults. Besides the fact that CAD is the primary cause of heart failure (HF), nevertheless DCM has recently became the most frequent diagnosis in patients referred for heart transplantation (HTX).
The etiology of DCM is largely heterogeneous. Despite enormous progress in the diagnosis and management of cardiovascular diseases, in many patients the causes of DCM remain unknown.
In developed countries ischemic heart disease (IHD) and myocardial infarction (MI) are the most common causes of HF, approximating 50-75% of all HF patients. Previously, even the term of ‘ischemic cardiomyopathy’ was extensively used in the literature but currently, this terminology is no longer recommended. Nevertheless, potentially reversible ischemia to the heart must always be sought actively, as effective therapy (medications and revascularization) can favorably alter patient’s outcome. Obviously, the presence of IHD and MI naturally excludes the diagnosis of cardiomyopathy at all. However, in the widely cited study by Felker et al., even after careful medical history and non-invasive examinations, the causative role of IHD in the development of HF, was ascertained only after coronary angiography in up to 7 percent of patients with initially unexplained DCM [3].
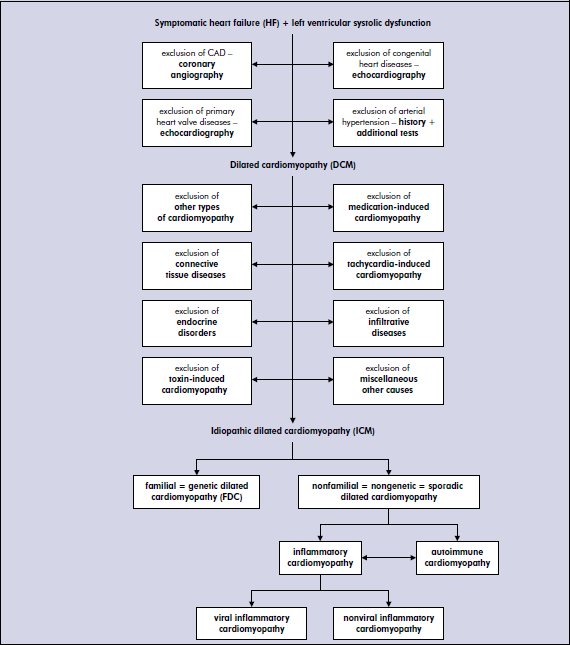
Other diagnosable causes of DCM, which always should be considering in the diagnostic process, include: remaining types of cardiomyopathies, which may either mimic or progress to DCM, connective tissue diseases, endocrinologic disorders, infiltrative diseases, medications and toxins, tachycardia-induced DCM and lastly miscellaneous causes. Most of those various diseases can be identified after careful medical history and basic laboratory and imaging studies (ECG, echocardiography). However, some disorders, especially infiltrative diseases may require more sophisticated examinations, such as laboratory tests, cardiac magnetic resonance (CMR), or the histopatopatological studies.
Once aforementioned diagnosable causes of DCM have been excluded, the etiology of DCM can be either genetic (familial) or non-genetic. The genetic nature of DCM is increasingly recognized, however at this stage only 35% of DCM patients have confirmed causative mutations. In the other spectrum are non-genetic causes, where persistent myocardial inflammation as a consequence of mycocarditis is probably most common [4].
Proposed etio-pathogentic (causative) diagnostic algorithm of dilated cardiomyopathy
Hypetrophic cardiomyopathy
Hypetrophic cardiomyopathy is characterized by otherwise unexplained left ventricular hypetrophy (LVH) with non-dilated ventricular chambers. Clinically, HCM is recognized by a maximal wall thickness ≥ 15 mm [5]. Of note, there is a considerable disagreement between European and American definition of HCM. In the European perspective, HCM is a largely heterogenous group of myocardial disorders that encompass not only sarcomeric protein diseases but also metabolic and infiltrative storage diseases. Such an approach is a reflection of everyday practice, when it is impossible to differentiate LVH caused by hypertrophy and cardiomyocyte disarray, typical for sarcomeric protein mutations, from interstitial (e.g. amyloidosis) or intracellular (e.g. Fabry disease) accumulation of metabolic substrates [1]. On the other hand, American guidelines endorse the concept that HCM is exclusively disease caused by the mutations of cardiac sarcomeric genes and in the case of the LVH that results from an infiltrative or metabolic causes, it cannot be termed as HCM [6]. The prevalence of LV hypetrophy, which is an essential manifestation of HCM phenotype, is about 0.2% (ie, 1:500) in the general population, HCM being a heterogenous group of diseases encompass various rare or even very rare conditions [5].
HCM attributable to sarcomeric protein mutations
HCM is a disease entity caused by autosomal dominant mutations in genes encoding contractile proteins of the cardiac sarcomere. At present, eleven mutant genes are associated with HCM, most commonly β-myosin heavy chain and myosin binding protein C. The remaining 9 genes account for far fewer cases of HCM and include troponin T and I, regulatory and essential myosin light chains, titin, α-myosin heavy chain, α-actin, α-tropomyosin, and muscle LIM protein. This genetic diversity is even more complicated by the intragenetic heterogeneity, with over 1400 individual mutations now identified. The majority are missense mutations but insertions, deletions or splice mutations are also commonly observed. As HCM is inherited in an autosmomal dominant pattern, the risk that an affected patient transmit disease to each offspring is 50% [5, 7, 8].
HCM attributable to non-sarcomeric protein mutations
In less than 10% of patients with echocardiographic phenotype of HCM, the disease is associated with other disorders, including infiltrative, metabolic, systemic, mitochondrial, and syndromic [1, 8, 9].
1. Infiltrative HCM can result from:
• glycogen storage diseases, such as:
– Pompe disease (caused by deficiency of α-1,4-glycosidase)
– mutation of the gene encoding the γ-2-regulatory subunit of the AMP-activated protein kinase (PRKAG2)
– Danon disease (mutation of the gene encoding lysosome-associated membrane protein-2 – LAMP-2)
– Forbes disease
• lysosomal storage disease, such as:
– Anderson-Fabry disease (caused by the deficiency of the lysosomal enzyme α-galactosisdase A),
– Hurler disease;
2. Metabolic myopathies, which are caused by ATP production and utilization defects:
• Disorders of fatty acid metabolism
• Carnitine deficiency
3. Systemic disease that can cause HCM:
• Pheochromocytoma
• Neurofibromatosis
• Lentiginosis
• Tuberus sclerosis
4. Mitochondrial cytopathies that include:
• Mutations encoding mitochondrial DNA – Kearns-Sayre syndrome
• Mutations of mitochondrial proteins associated with ATP electron transport chain enzyme
5. Syndromic HCM that include:
• Noonan syndrome
• LEOPARD syndrome
• Fredreich’s ataxia
• Beckwith-Weidermann syndrome
• Swyer’s syndrome
Moreover, two the most common conditions, such as hypertensive heart disease and the physiologic remodeling associated with athletic training (“athlete’s heart”), should always be taken into account. Young age, history of training, and maximal wall thickness in the modest range of 13 to 15 mm, strongly suggests “athlete’s heart” [8].
Restrictive cardiomyopathy
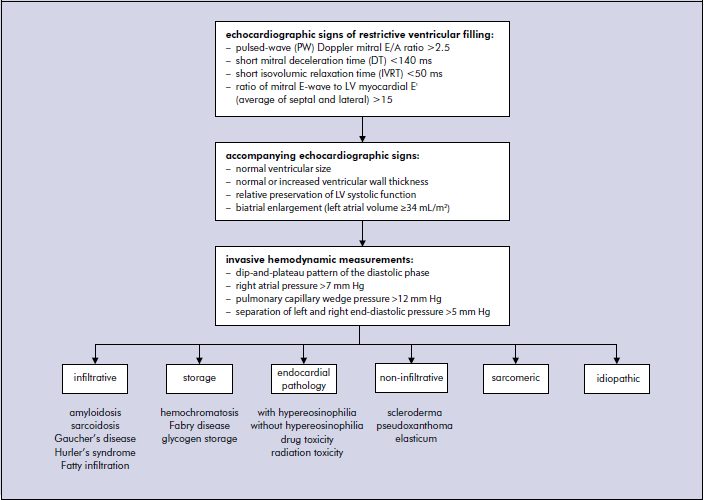
Restrictive cardiomyopathy (RCM) is an uncommon, heterogeneous group of heart muscle disorders that is characterized with an impaired ventricular filling, with normal or even decreased ventricular volumes [1]. This leads to advanced diastolic dysfunction (DD) with relative preservation of systolic function. However, it is not true that systolic function is completely normal in RCM as usually only short axis contractility is preserved, whereas long axis may be severely depressed. Depending on the underlying etiology ventricular wall thickness may be normal or increased. Atrias are usually severely dilated due to increased ventricular resistance they constantly face in each diastole. The precise epidemiology of RCM is unknown but true RCM is a rare disease. Importantly, RCM is a diagnosis of exclusion because restrictive physiology is typically observed in numerous other cardiac disorders, including end-stage HCM or early stages of DCM. The majority of RCM are secondary to systemic disorders, such as amyloidosis, sarcoidosis, scleroderma, haemochromatosis, eosinophilic heart disease, or as a result of radiation therapy. The rarest diagnosis of idiopathic RCM is only made after active exclusion of presented below more common causes of RCM [10].
• Infiltrative disorders, such as amyloidosis (familial – transthyretin or apolipoprotein, and non-familial – AL/prealbumin), sarcoidosis, Gaucher disease, Hurler’s disease, and fatty infiltration.• Storage disease, such as hemochromatosis, Fabry disease, and glycogen storage diseases.
• Non-infiltrative disorders, such as scleroderma, pseudoxanthoma elasticum.
• Sarcomere protein genes mutations – as in other types of cardiomyopathies, some proportion of RCM patients have defective contractile proteins, caused by mutations.
• Disorders that cause endocardial pathology (fibrosis, fibroelastosis, thrombosis) that are sub-classified according to the presence of eosinophilia into endomyocardial diseases with hypereosinophilia, also known as hypereosinophilic syndromes (HES), and endomyocardial diseases without hypereosinophila or endomyocardial fibrosis (EMF). Acquired forms of endomyocardial fibrosis can be caused by parasitic infection, drugs such as methysergide, serotonin, buslfan, nutritional factors, and radiation toxicity.
Morover, RCM has to be always distinguished from constrictive pericarditis, which presents with similar impairment of diastolic function. Patient’s history my be crucial as the commonest causes of constrictive pericarditis are open-heart surgery, radiation therapy and uremia. Computed tomography and cardiac magnetic resonance are useful to define pericardial thickness and the presence of calcification. However, echocardiography with Doppler assessment of mitral inflow during the respiratory cycle play the decisive role. In RCM there are no changes of trans-mitral and trans-tricuspid inflow velocities neither during inspiration nor expiration. On the contrary, in constrictive pericarditis the trans-mitral velocities are reduced while the tricuspid velocities are inreased in deep inspiration, while the opposite happens during expiration [11].
The proposed diagnostic algorithm of RCM:
References
1.Elliot, Anderson B, Arbustini A, Bilińska Z, Cecchi F, Charron P, Dubourg O, Kuhl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, and Keren A. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2007; 29: 270-7.
2.Codd MB, Sugrue DD, Gersh BJ, et al. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975-1984. Circulation 1989; 80: 564-72.
3.Felker GM, Thompson RE, Hare JM et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 200; 342: 1077-84.
4.Maisch B, Bultman B, Factor S. World Heart Federation consensus conference’s definition on inflammatory cardiomyopathy (myocarditis): report from two expert committees on histology and viral cardiomyopathy. Heartbeat 1999; 4; 3-4.
5.Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH 3rd, Spirito P, Ten Cate FJ, and Wigle ED. American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. Eur Heart J 2003; 24: 1965-91.
6.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, and Young JB. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006; 113: 1807-16.
7.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 124: 2761-96.
8.Elliot P, Lambiase P, Kumar D [edited by]. Inherited Cardiac Disease. Oxford University Press 2011.
9.Kumar D, Elliot P [edited by]. Principles and practice of clinical cardiovascular genetic. Oxford University Press 2010.
10.Mogensen J, Arbustini E. Restrictive cardiomyopathy. Curr Opin Cardiol 2009; 24: 214-20.
11.Maisch B, Seferović PM, Ristić AD, Erbel R, Rienmüller R, Adler Y, Tomkowski WZ, Thiene G, Yacoub MH; Task Force on the Diagnosis and Management of Pricardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European society of cardiology. Eur Heart J 2004; 25: 587-610.
III. Rare diseases of the heart (cardiomyopathies)
- (III-3A.3) �32-year-old-patient with cardiac sarcoidosis Justyna Błaut-Jurkowska MD, Klaudia Knap MD, Prof. Maria Olszowska MD, PhD, Prof. Piotr Podolec MD, PhD
- (III-3A.2) A patient with multiple myeloma and suspected cardiac amyloidosis Piotr Liszniański MD, Jacek Nowak MD, PhD
- (III.5A) Hypertrophic cardiomyopathy with left ventricular outflow tract obstruction Natalia Dłużniewska MD; Leszek Drabik MD, PhD; prof. Maria Olszowska MD, PhD; Prof. Piotr Podolec MD, PhD
- (III-1A.5.o) 49-year old female with cardiac failure and mitochondrial myopathy M. Dzieciuch-Rojek, P. Rubiś MD, PhD, S. Wiśniowska-Śmiałek MD, J. Stępniewski MD, D. Kudliński MD, A. Leśniak-Sobelga MD, PhD, M. Kostkiewicz MD, PhD, Prof. P. Podolec MD, PhD
- (III-2B ) 61-year-old male with hypertrophic cardiomyopathy Joanna Łuszczak MD, Agnieszka Żygadło MD, Lidia Tomkiewicz-Pająk MD, PhD, Prof. Maria Olszowska MD, PhD, Prof. Piotr Podolec MD, PhD
- (III-5A.1.o) 27-year-old patient with �Left Ventricular Noncompaction� and �impaired systolic function Jakub Stępniewski MD, Paweł Prochownik, Grzegorz Kopeć MD, PhD
- (III-2B.2a) Induction of intraventricular pressure gradient during replacement enzyme therapy (ERT) Paweł Petkow-Dimitrow, Danuta Sorysz 2nd Deparment of Cardiology CMUJ
- (III-1A.1) 24 years old male with ventricular arrhythmia and family history of dilated cardiomyopathy and sudden cardiac deaths. Jakub Stępniewski MD, Paweł Rubiś MD, PhD, Agata Leśniak-Sobelga MD PhD, Magdalena Kostkiewicz MD, PhD.
- (III-2B) Hypertrophic cardiomyopathy (HCM) in complex with chronic respiratory failure caused by kyphoscoliosis Prof. R.Benetis MD, PhD, Prof.; E.Ereminienė MD, PhD, Prof.; N.Stoškutė, Assist. Prof.; S.Miliauskas MD, PhD, Prof.; R.Ordienė, MD,.; D.Hoppenot, M.D.PhD.
- (III-3E) 33- year old male with restrictive cardiomyopathy and peripheral muscle weakness Jakub Stępniewski MD, Hanna Dziedzic-Oleksy MD, Grzegorz Kopeć MD. PhD, Piotr Wilkołek MD. PhD, Prof. Piotr Podolec MD. PhD.

